For most of us, cancer is a familiar word since it may affect a relative, friend, colleague, or domestic animals such as dogs or cats1. We are also aware of that important discovery achieved thanks to the use of mice, rats, or pigs as cancer models2. But does cancer affect other organisms of the animal kingdom?
Do wild animals such as leopards, horses, and kangaroos get cancer?
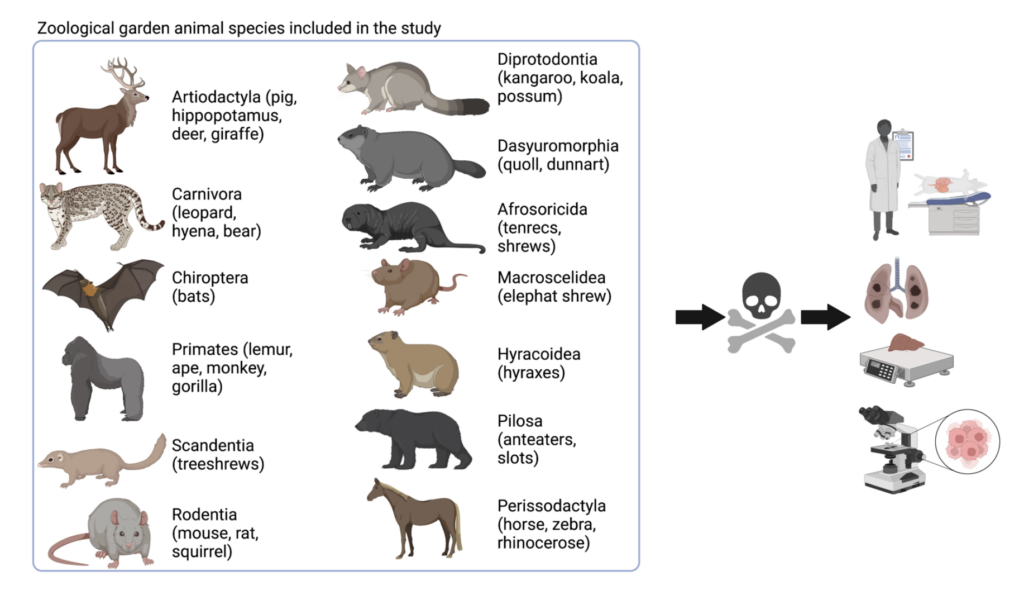
Investigating animals living in their natural habitat is challenging since they are hard to identify individually and follow for long periods. Zoos represent a good alternative for those studies since they keep track of animals’ activities and, most importantly, medical records and necropsy information. Upon the death of a zoo animal, veterinarians routinely examine them to determine the cause of death. By observing how the animal’s body is, weighing organs that may be enlarged by visible cancer, and looking under the microscope to magnify areas of different organs and tissues, veterinarians can assess if tumors were present. The International Species Information System Species3603 records zoo data, including findings upon dead organisms, to get an orientation on cancer incidence in many species.
Zoo animal death record: approximation to cancer incidence in wild species
To answer how frequent cancer is in zoo animals, the authors of a recent study published in Nature 4 (open access), Orsolya Vincze and colleagues analyzed zoos’ databases by looking at the death reports comprising 110,148 mammals from 191 species (Figure 1), including giraffes (Artiodactyla), leopards (Carnivora), mice (Rodentia) and anteaters (Pilosa). They evaluated cancer risk by counting how many animals get tumors and dividing that amount by the number of animals analyzed. For example, 0% cancer risk means that none of the examined animals got cancer; 10% cancer risk means that for a group of 100, 10 analyzed animals got cancer; or 100% cancer risk implies that all of the analyzed animals got cancer.
In around a quarter of the analyzed species, the blackbuck and the Patagonian mara, cancer was absent or barely detected. Most of the species got a cancer risk ranging from 0% to 10%. The highest cancer rate was found in kowari: tumors were observed in more than half of the examined animals.
Nutrition and cancer risk: is there a link in mammals?
After classifying animals into related organisms (orders), the scientists observed that ruminants, a subtype of Artiodactyla, presented fewer cancer lesions. On the contrary, in Carnivora, the largest amount of cancer lesions were detected (Figure 2). A remarkable difference among those animal groups is their diet. The food requirements of ruminants derive from plants, which are fermented in a specialized stomach prior to digestion. That is an essential difference in respect to carnivores, which acquire food from animal products, usually raw meat.
Compared to ruminants, carnivores have less microbiome diversity and due to their high meat consumption, they may get more harmful pathogens that may be linked to cancer development. Since those findings were found in zoo animals, it may be that carnivores are limited to the amount and type of exercise they would do in their wild habitat.
Cancer incidence, life span, and body size
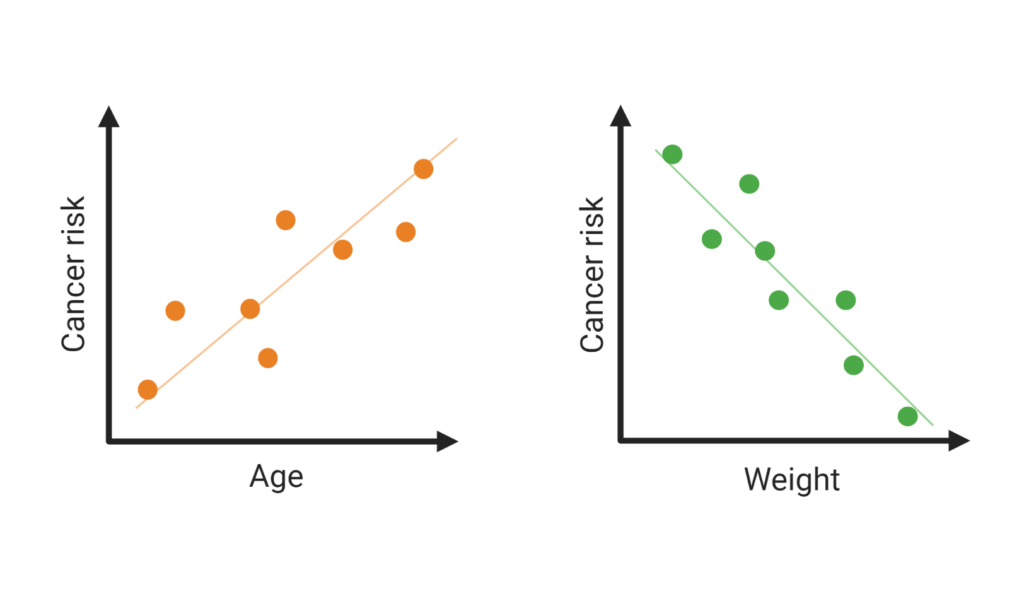
One would expect that cancer is more frequent in big and long-lived animals since those organisms have more cells. Thus one would think that the probability that a cell loses control over its death and keeps proliferating is higher, which could easily lead to cancer. Scientific observations have demonstrated that this thought does not hold and have termed this event as “Peto’s Paradox”5. The findings of this study indeed provide evidence that cancer risk across species increases with longevity, and large-bodied animals have a decreased rate of cancer risk (Figure 3). This paradox suggests that other mechanisms have a higher impact rather than cell amount and that there must be different ways to improve the control over potentially harmful own cells.
Scientific research: observe, analyze, and complement other studies
Science does not depend on a single team, it is multiple teamwork and this research exemplified this. It is rewarding for the community that the use of generated and well-recorded data provided by zoos helps in getting a broader animal perspective on cancer. This study complements previous ones and shows that cancer is a problem spread among mammals, albeit affecting at different percentages6.
Nature has been dealing with this disease in different ways, which may differ according to the specie7. Knowing the underlying reasons for an animal’s death may help improve the handling and maintenance conditions for that species. Knowledge is a powerful tool. Studying and comparing how ruminants and carnivores are and looking at their similarities and differences may highlight cancer suppression mechanisms that can be extrapolated to human cancer treatment.
References
- Thomas, F., Giraudeau, M., Dheilly, N. M., Gouzerh, F., Boutry, J., Beckmann, C., Biro, P. A., Hamede, R., Abadie, J., Labrut, S., Bieuville, M., Misse, D., Bramwell, G., Schultz, A., le Loc’h, G., Vincze, O., Roche, B., Renaud, F., Russell, T., & Ujvari, B. (2020). Rare and unique adaptations to cancer in domesticated species: An untapped resource? Evolutionary Applications, 13(7), 1605–1614. https://doi.org/10.1111/eva.12920
- Schachtschneider, K. M., Schwind, R. M., Newson, J., Kinachtchouk, N., Rizko, M., Mendoza-Elias, N., Grippo, P., Principe, D. R., Park, A., Overgaard, N. H., Jungersen, G., Garcia, K. D., Maker, A. V., Rund, L. A., Ozer, H., Gaba, R. C., & Schook, L. B. (2017). The Oncopig Cancer Model: An Innovative Large Animal Translational Oncology Platform. Frontiers in Oncology, 7. https://doi.org/10.3389/fonc.2017.00190
- Species360. (2019, November 6). https://www.species360.org/
- Vincze, O., Colchero, F., Lemaître, J. F., Conde, D. A., Pavard, S., Bieuville, M., Urrutia, A. O., Ujvari, B., Boddy, A. M., Maley, C. C., Thomas, F., & Giraudeau, M. (2021). Cancer risk across mammals. Nature, 601(7892), 263–267. https://doi.org/10.1038/s41586-021-04224-5
- Peto, R., Hiatt, H. H., Watson, J. D., & Winsten, J. A. (1977). Origins of human cancer. by. Hiatt, HH, Watson, 3, 1403.
- Albuquerque, T. A. F., Drummond Do Val, L., Doherty, A., & de Magalhães, J. P. (2018). From humans to hydra: patterns of cancer across the tree of life. Biological Reviews, 93(3), 1715–1734. https://doi.org/10.1111/brv.12415
- Seluanov, A., Gladyshev, V. N., Vijg, J., & Gorbunova, V. (2018). Mechanisms of cancer resistance in long-lived mammals. Nature Reviews Cancer, 18(7), 433–441. https://doi.org/10.1038/s41568-018-0004-9
Featured illustration: Sofia Polcownuk